Consumables Sanity 2.0 – High Risk HPV Genotyping kit (24 tests)
€ 422,29 € 349,00 ex. VAT
The High Risk HPV Genotyping Kit (Abbreviation: Sanity 2.0 hrHPV) is a qualitative in vitro test used for HPV genotyping and cervical cancer screening using cervical cells collected by swab, TCT or LCT. This test utilizes Polymerase Chain Reaction (PCR) for DNA amplification and nucleic acid hybridization for the detection of high-risk HPV types in one reaction. This test specifically identifies 14 high-risk HPV types, including HPV-16, HPV-18, HPV-31, HPV-33, HPV-35, HPV-39, HPV-45, HPV-51, HPV-52, HPV-56, HPV-58, HPV-59, HPV-66, and HPV-68.
Available on backorder
The High Risk HPV Genotyping Kit (Abbreviation: Sanity 2.0 hrHPV) is a qualitative in vitro test used for HPV genotyping and cervical cancer screening using cervical cells collected by swab, TCT or LCT. This test utilizes Polymerase Chain Reaction (PCR) for DNA amplification and nucleic acid hybridization for the detection of high-risk HPV types in one reaction. This test specifically identifies 14 high-risk HPV types, including HPV-16, HPV-18, HPV-31, HPV-33, HPV-35, HPV-39, HPV-45, HPV-51, HPV-52, HPV-56, HPV-58, HPV-59, HPV-66, and HPV-68.
The Sanity 2.0 hrHPV is based on the technology of multi-color melting curve analysis (MMCA). HPV genotypes are identified by the different Tm values of hybridizing PCR products with the fluorescent probes.
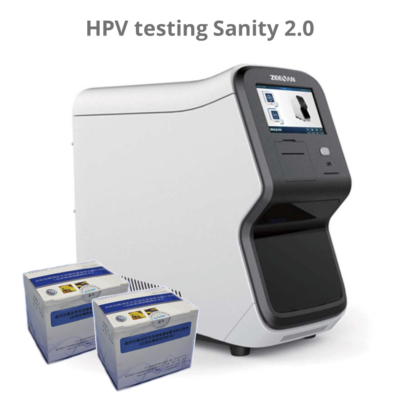
The hrHPV genotyping kit should be used in combination with the Sanity 2.0 and the HPV DNA extraction kit.
Technical details:
- Limit of Detection (LOD): 500 copies per reaction
- Detection specificity: > 99%
- Clinical sensitivity: >95%
- Repeatability & precision: the coefficient of variation (CV) of the Tm values is no higher than 5%.
About the Sanity 2.0:
The Automatic Medical PCR Analysis System Sanity 2.0 is an automated Point-of-Care (POC) system that integrates specimen extraction and purification, PCR amplification, signal generation and optical detection. Once the user loads the specimen into the extraction loading well and starts the run, all other operations are automated on the instrument. The software preloaded in the instrument will collect and analyze data, and automatically generate interpretation for test reports at the end of the run. The system utilizes magnetic particle technology for automated isolation and purification of nucleic acid from saliva, bronchoalveolar lavage fluid (BALF), sputum, swabs, etc.
The product is intended to be used by professional users only.
The Automatic Medical POC PCR Analysis System Sanity 2.0 performs all steps of the sample preparation procedure for 4 samples in a single run.
Storage guidelines:
The Sanity 2.0 H-HPV should be stored at 2~8 ℃ away from direct (sun)light. Production date and expiration date are shown on the package label.