Our SARS-CoV-2 Antigen Rapid Test has been added to the European Union’s HSC list!
Our SARS-CoV-2 Antigen Rapid Test has been added to the latest version of the “Common list of Covid-19 rapid antigen tests”. This recommendation list has been developed by the European Union’s Health Security Committee (HSC) and was last updated on October 20th, 2021.
The HSC was set up as an advisory board in 2001 following the request of the EU Health Ministers. Since then, they have been involved in reinforcing the coordination of health related information on national preparedness activities.
As can be read in the full report, “The Health Security Committee (HSC) agreed on 17 September 2020 on Recommendations for a common EU testing approach for COVID-19, setting out various actions for consideration by countries when updating or adapting their testing strategies.”.
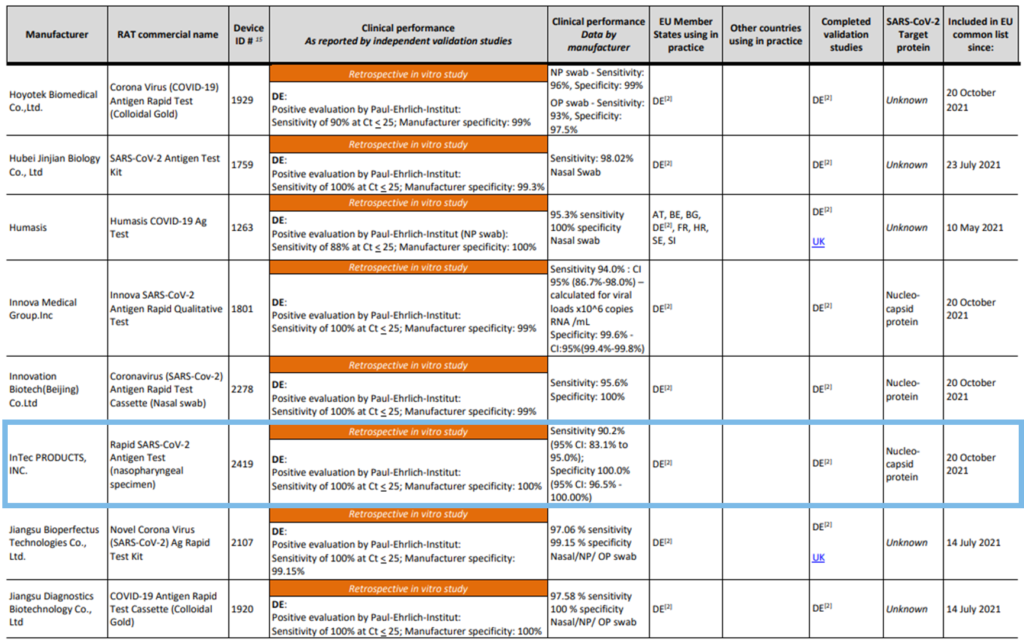
The results of the evaluation of our SARS-CoV-2 Antigen Rapid Test, manufactured by InTec, are shown on page 26:
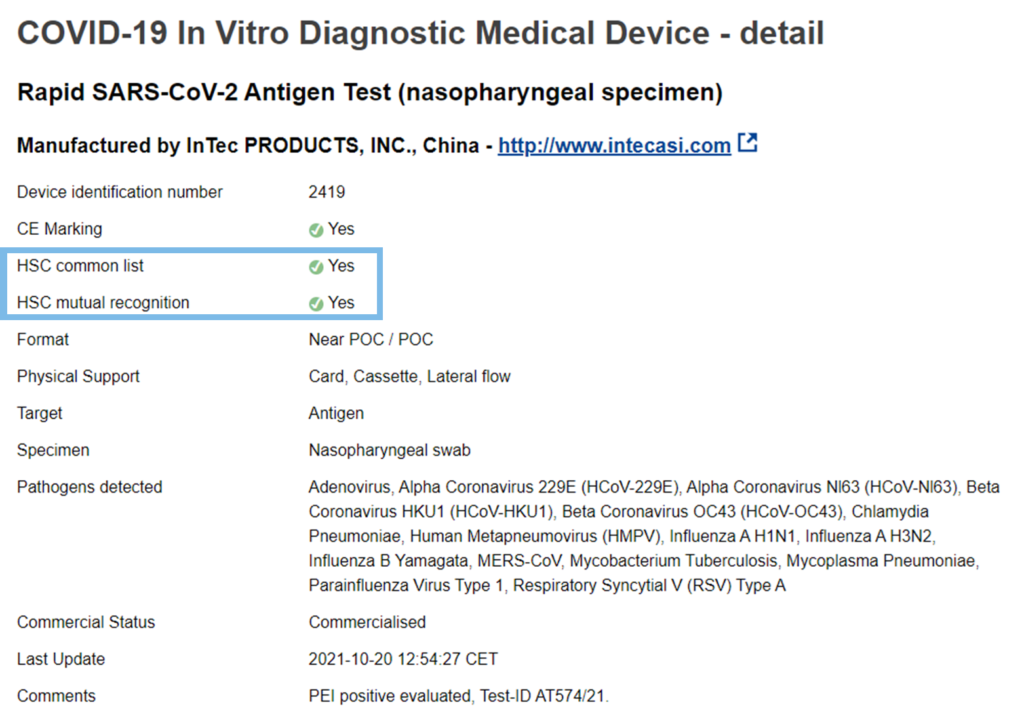
A more detailed description of our test can be found online in the “COVID-19 In Vitro Diagnostic Devices and Test Methods Database“:
Interested to learn more? Please contact us!