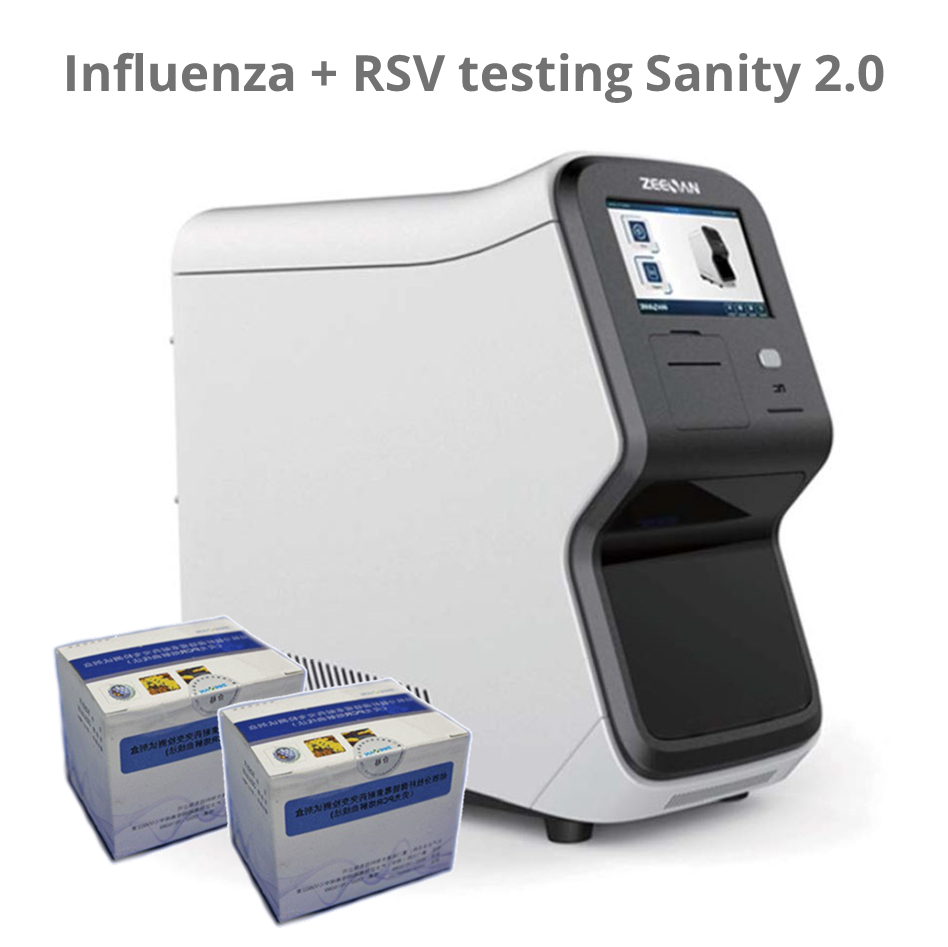
Sanity 2.0 – Respiratory pathogen panel 12; RPP12; 24 testen
€ 1.087,79 € 899,00 ex. VAT
The Sanity 2.0 Respiratory Pathogen Panel RPP12 is a multiplex real-time, reverse transcriptase polymerase chain reaction (RT-PCR) test intended for the in vitro qualitative detection and differentiation of viral RNA in nasopharyngeal swab (NPS), oropharyngeal swab (OPS) and sputum specimens obtained from individuals suspected of respiratory tract infections.
Op voorraad (kan nabesteld worden)
The product is intended for qualitative detection of 12 kinds of clinically common respiratory pathogens in nasopharyngeal
swab, oropharyngeal swab, sputum, saliva and bronchoalveolar lavage fluid, including Influenza virus A (covering
H1N1-2009, H7N9, H1N1, H3N2, H5N1), Influenza virus B (covering Victoria strain and Yamagata strain), Adenovirus
(covering group B, C and E), Bocavirus, Rhinovirus/Enterovirus, Parainfluenza Virus (covering type 1, 2, 3 and 4),
Coronavirus (covering type 229E, OC43, NL63 and HKU1), Respiratory Syncytial Virus (covering group A and B), Human
Metapneumovirus, Mycoplasma pneumoniae, Chlamydia pneumoniae and Bordetella pertussis. The subtypes of pathogens
are not classified.
The kit should be operated on Automatic Medical PCR Analysis System Sanity 2.0 System; Xiamen Zeesan Biotech Co., Ltd.) which has FAM, HEX, ROX and CY5 detection channels, in combination with the pathogen nucleid acid extraction kit.
About the Sanity 2.0:
The Automatic Medical PCR Analysis System Sanity 2.0 is an automated Point-of-Care (POC) system that integrates specimen extraction and purification, PCR amplification, signal generation and optical detection. Once the user loads the specimen into the extraction loading well and starts the run, all other operations are automated on the instrument. The software preloaded in the instrument will collect and analyze data, and automatically generate interpretation for test reports at the end of the run. The system utilizes magnetic particle technology for automated isolation and purification of nucleic acid from saliva, bronchoalveolar lavage fluid (BALF), sputum, swabs, etc.
The product is intended to be used by professional users only.
The Automatic Medical POC PCR Analysis System Sanity 2.0 performs all steps of the sample preparation procedure for 4 samples in a single run.
SARS-CoV-2 test kits:
Apart from the respiratory panel kit, we also have several kits available for the detection of SARS-CoV-2:
- Automatic Medical PCRAnalysis System(Sanity 2.0 System; Xiamen Zeesan Biotech Co., Ltd.)
- Disposable powder-free gloves, lab coat and protective goggles
- Adjustable pipettes and sterile filtered pipette tips
- Sanity 2.0-Pathogen Nucleic Acid Extraction Kit